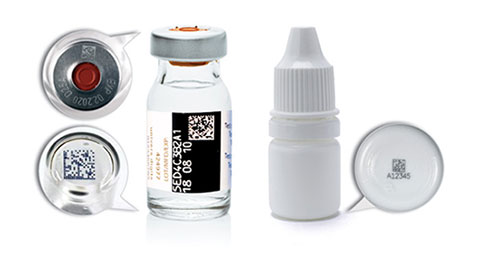
Overcoming the
Challenges in Serialization
and Track & Trace Programs
Have you taken all the necessary measures to comply with the new regulations in time?










Overcoming the
Challenges in Serialization
and Track & Trace Programs










1030 Vienna | Am Stadtpark 1
Tel.: +43 (0)1 71 700 15000
Email: [email protected]
Website: http://www.hilton.com/
* Car Parking: EUR 34,20 (day); ** Guest rooms start at EUR 189,00
| Time | Activity | Topic |
|---|---|---|
| 09:00 | Registrations | |
| 09:30 | Welcome & Introductions | |
| 09:45 | Presentation: Tania Snioch, GS1 (Director Healthcare) |
GS1 and Solution Providers Helping Healthcare Stakeholders |
| 10:30 | Presentation: Jean-Marc-Bobee, Sanofi (Director Industrial Anti-Counterfeit Strategy) |
Anti-Counterfeiting Technological Strategies: How Technology Can Help To Fight Counterfeits and Improve Patient Safety? |
| 11:15 | Coffee Break & Networking | |
| 11:45 | Presentation: Heidi Vanheerswynghels, Videojet Technologies (Pharma Sales Manager) |
Implementing Best Practice Coding Solutions for Track & Trace |
| 12:20 | Presentation: Michel Bullen, Optel Vision (Track & Trace Solution Manager) |
Packaging Solutions - CMO Specific T&T Packaging Solutions, Roll-Out Experience & Learnings |
| 13:00 | Lunch & Networking | |
| 14:00 | Presentation: Sean Mulholland, Almac Group (Technical Quality Manager) |
Serialization - CMO Case Study |
| 14:45 | Presentation: Matthias Korbl, Arena Pharma (Head of Supply Chain) |
From Switzerland to South Korea And Beyond – Implementing Serialization in a Swiss Mid-Size Pharma Company |
| 15:30 | Coffee Break & Networking | |
| 16:00 | Round Table Discussions Sponsored by TraceLink |
(Selection of Topics During the Event Day) 1. Internal / Site Serialization Program Strategy 2. Packaging Line Implementations & Artwork Changes 3. Serialization of IT Systems: Implementation & Integration 4. CMO Serialization Readiness & Support Strategy 5. IT Connectivity / Integration With CMOs & Customers 6. ROI / Value Beyond Compliance With Track & Trace 7. Process & Change Management: Warehousing, QA, Exception Management 8. Master Data Management Strategy, Systems & Processes 9. Pilot Projects: IT Proof of Concepts & Packaging Line Pilots |
| 17:45 | Closing Remarks | |
| 18:00 | Dinner |

Jean-Marc Bobee is Director, “Anti-counterfeiting Strategy” at Sanofi, Industrial Affairs. In this capacity, he is in charge of the projects related to medicines protection and traceability. Jean-Marc is also the Director of the global Sanofi serialization program. Jean-Marc is Doctor of Pharmacy and holds a Master Degree in Industrial Pharmacy from Paris University.
He has more than 30 years extensive experience in international products development (galenics, analytics & technology transfer) and management of Industrial Operations Interfaces (risk assessment & strategy).
Jean-Marc was also the chairman of the EFPIA project on codification and identification of pharmaceuticals in Europe from June 2007 to May 2010.

Matthias Korbl has more than 19 years of work experience in supply chain management and business reengineering projects. He started his career in consultancy and is highly experienced in design and operation of integrated ERP systems. Matthias managed multi-national projects of various scales in process, repetitive and discrete manufacturing.
He joined the business side in 2007 to work for pharmaceutical companies in the originator and generics market. He worked in international assignments in Africa, North America and Europe and possesses a profound knowledge of planning processes in regulated industries as well as of operational processes in commercial and manufacturing sites.
Matthias is in charge at Arena of customer service, purchasing, planning, data management and project management.

Tania Snioch is Director Healthcare at GS1 Global Office. Prior to starting at GS1, Tania worked for the Australian GS1 organisation, GS1 Australia, for nearly 15 years, holding various roles in that time.
At GS1 Australia, Tania and her team assisted the Healthcare industry to implement the GS1 System to improve patient safety and increase supply chain efficiency. Tania worked with Australian state and territory governments and federal regulators.
In her role at GS1 Global Office, Tania supports the GS1 community by pro-actively responding to specific business requirements from industry sectors with the aim to enhance the level of GS1 System adoption.

Sean Mulholland has been for over 22 years an integral part of Almac Group, an established contract development and manufacturing organisation, where he has held various technical roles and managed several teams, including Quality Compliance and Product Quality.
Currently Technical Quality Manager, Sean and his team are in charge of key tasks such as developing processes and procedures to support new system/product introductions, conducting qualification and validation activities, and providing quality support for business and continuous improvement projects.
Considered Almac’s SME expert in areas like process validation and Serialization, Sean has been a member of these project teams for over 7 years, participating in system design and qualification, as well as validation of software, infrastructure and hardware. His ongoing involvement also includes the implementation of commercial Serialization processes.

Heidi Vanheerswynghels is the pharma EMEA sales manager at Videojet Technologies. She leads a defined group of the company’s global pharmaceutical key accounts and carries responsibility for all medical and pharmaceutical end user business within EMEA. Taking a central role in sales growth with the dedicated pharma team, she also supports the Videojet regional and local sales teams. In addition, Heidi works closely with the Videojet marketing team building a strong Videojet brand and coordinates with the OEM organization and product development teams.
Heidi has been focusing on pharma and medical business for more than 10 years and brings significant experience in the medical and pharmaceutical packaging industry, having held various roles as Global Strategic Account Manager Pharma and Key Account Manager Healthcare.

Michael Bullen has nearly 15 years experience in vision and Serialization and has worked for several world-renowned companies in the pharmaceutical industry.
Over the years, Michael has focused on various aspects of vision and Track & Trace systems, including engineering, product management and project management. Namely, he specializes in camera-based inspection of products as well as Serialization for pharmaceutical packaging. In addition, Michael has extensive knowledge of IT and various platforms.
As Track & Trace Solution Manager, Michael currently works with Optel Vision's European team, using his combined expertise to propose the best possible overall solution for customers' specific requirements and packaging lines.
| Registration Type | Price |
| Registration Fee (Per Person / including VAT) | EUR 199,00 |